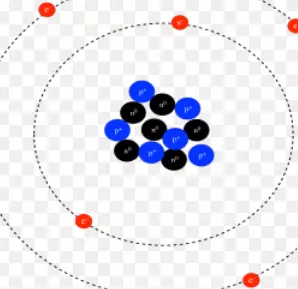
Where Are the Electrons Located in an Atom?
You might be wondering – where are the electrons located in an atomic nucleus? The atom has two parts – a nucleus and an electron shell. The nucleus is the center of the atom and contains the protons and neutrons, while the electron shells are on the outer part of the atom and contain only the electrons. This article will cover both.
Nucleus
The electrons are tiny particles that orbit the atomic nucleus. We cannot see them at any given moment, but scientists have figured out that these particles are in well-defined volumes around the center of the atom. Electrons are particles and waves that behave differently at different points in space, so they can’t be seen directly in any specific place. Instead, we can only determine where they’re likely to be by probability.
The positive charge of the electron attracts it to the nucleus, where the proton is located. The electron is more than 1800 times smaller than the proton, so its attraction is powerful. But there are also neutrons in an atom, which do not have an electric charge. The number of neutrons in an atom affects its mass and radioactivity. Quarks, which are extremely small and almost impossible to detect, are tiny particles that are not easily detected by human eyes. They were discovered in 1964 by Murray Gell-Mann and were named up, down, charm, and strange.
When you’re wondering where the electrons are in an atom, it’s essential to realize that the atom has two parts: the nucleus and the orbitals. The nucleus is the center of an atom, and the orbitals are the surrounding space. The orbitals are different shapes depending on how much energy they contain. This information is necessary to understand the interactions between different atom parts.
Electron shell
The electron shell in an atom is the space around the nucleus where a pair of electrons can be found. The shells are classified into three types: s, p, and d. The n=1 shell contains only s and p orbitals, while the n=2 and n=3 shells contain all four types of orbitals. It is helpful to define the atomic structure to understand better how an atom works.
The electrons in an atom orbit around the atomic nucleus at different energy levels. The energy level increases the further an electron is from the nucleus. Each electron orbit has a quantum number, or n, which is assigned to it. The n-shell has subshells corresponding to different rates of rotation, orientations of orbitals, and spin directions of the electrons. The higher the n, the greater the number of electrons.
The number of electrons in an atom varies depending on the type of atom. The s-shell, for example, contains two electrons. The second column indicates the maximum number of electrons a particular subshell can hold. This information helps identify the exact energy of electrons within an atom. A typical atom has one or two subshells. The process of filling an atom with electrons continues from lower to higher energy subshells.
Atoms contain negatively charged subatomic particles.
There are two types of negative-charged subatomic particles: electrons and protons. Electrons are negatively charged and are usually found outside the nucleus. The attraction of a positively charged nucleus causes electrons to remain in atoms. Hence, a neutral atom must contain the same number of electrons and protons as its nucleus. However, the existence of both types of subatomic particles in atoms does not mean it is always neutral.
Protons and neutrons are positively charged subatomic particles found inside the nucleus of an atom. These particles are the main constituents of the atom and have the same mass as protons. The number of electrons does not contribute to the overall mass of an atom. Therefore, an atom will always have equal amounts of protons and electrons. If you add more electrons to an atom, it will be heavier.
The smallest unit of matter is an atom. Atoms are made up of positively and negatively charged subatomic particles, which make up the nucleus. Protons are positively charged, while electrons have a negative charge. Protons are located in the center of the atom, while electrons are located outside the nucleus. Both positively and negatively charged subatomic particles are located at different energy levels around the nucleus.
Atoms behave in discrete packets.
As the popularity of quantum theory grew, it became clear that atoms didn’t behave in continuous forms but instead in discrete packets. While quantum theory can explain the physical properties of atoms, it can’t explain how discrete energy packets form. This led to a new model proposed by Neils Bohr in 1913. Bohr showed that atoms are composed of a positively charged nucleus and electrons that orbit around it. Each electron has a specific energy level, just as a planet orbits the Sun.
The concept of discrete packets of energy is based on the fact that electrons exist in a finite amount of energy. Atoms exist in discrete energy levels, and electrons can move between them. This is possible because of the energy imparted by force or heated atoms. This energy allows electrons to be excited, thereby causing them to move from one energy level to another. The effect is called a “quantum leap.”
Chemical properties of atoms
Atoms are neutral particles composed of protons and electrons. The number of protons and electrons in an atom determines the element’s identity, and the valence electrons are the outermost particles. Valence electrons have a specific configuration and take part in chemical reactions. There are five to seven valence electrons per atom, and the number of protons in an atom determines the element’s chemical properties.
Electronegativity refers to the attraction of electrons to nuclei. Atoms with a higher electronegativity attract more electrons than those with a lower value. Fluorine, for example, is the most electronegative element, while cesium, francium, and nitrogen are the least electronegative. Similarly, the ionization potential refers to the energy required to remove an electron from a neutral atom.
Super-light electrons surround the atomic center. These electrons behave like particles one moment and as waves the next. As such, most physical properties of an atom depend on its electron number and density. These physical properties determine the atom’s behavior concerning other atoms. Despite the apparent simplicity of atoms, many questions remain about them. This article will look at some of the most common and essential atomic properties.
Bonding between atoms
What is the purpose of chemical bonding? Bonding between atoms occurs when the interaction between two atoms leads to an aggregate with a specific structure or properties. It’s what makes some molecules or ions stable. In chemical reactions, atoms are attracted to one another, and the attractive forces between them lower the total potential energy of the compound. These attractive forces are responsible for determining the characteristic properties of metals.
The covalent bond between two atoms is a simple example. One carbon atom forms four covalent bonds with four hydrogen atoms. Each atom shares a pair of electrons with the other. The carbon atom is more electronegative than hydrogen, so the two share electrons equally. This gives methane molecules their four covalent bonds. The number of electrons shared between the two atoms determines the type of covalent bond.
Understanding the nature of chemical bonds helps to understand the electronic structure of an atom. An atom’s electrons are arranged around its central nucleus. The atoms share the outermost electrons of a compound, and those atoms take up the other atoms’ electrons. In addition, a compound’s atoms are bonded by repulsion between two molecules.
Number of protons
The number of protons in an atom is one of the elements that scientists use to identify elements. For example, hydrogen atoms have one proton, while oxygen atoms have eight protons. Counting the protons of atoms helps scientists determine what elements are similar and what elements are different. Atoms with one proton are called hydrogen atoms, while those with two protons are called helium atoms. Atoms with four or more protons are classified as beryllium atoms, while those with six or more are called carbon atoms.
The atomic number is the number of protons in an atom, also called the atomic mass. The total positive charge of an atom is the atomic mass, and its number determines the mass of the atom. To determine the mass of an atom, scientists must first measure the photon energy of the atom, which is related to the square root of the atomic charge. This was done by Henry Moseley, a chemist who measured the energy of photons of many elements.
Number of electrons
The number of protons and electrons in an atom is equal. A hydrogen atom has one electron outside its nucleus, while a helium atom has two. The protons and electrons of an atom will never change, and the atomic number will remain the same unless the nucleus decays or is bombarded. There are a few examples of this, which are described below.
The number of protons and electrons in an atom is determined by dividing the atomic number by the number of electrons. When you have a positive ion, there are more protons than electrons. For example, calcium has a positive charge of +2 and an atomic number of 20. In contrast, calcium has 18 electrons. As we can see, electrons and protons have opposite charges. This is what helps keep the atom neutral.
An atom’s atomic weight determines the number of electrons. A neutral atom with no charge at all will contain the same number of protons. This makes it easier to identify, although more challenging to manipulate. For example, a lithium atom has three protons, while a beryllium atom has four protons. The atomic weights of these atoms are similar.